The RNA-Seq quantitation pipeline
The RNA-Seq pipeline is a specialised quantitation methods which is useful
for the analysis of RNA-Seq expression data in genomes containing spliced
transcripts.
The pipeline generates a set of probes covering every transcript in the genome, but
then quantitates each of these based on the number of reads falling within the
exons of those transcripts - ignoring any reads found in introns. This method of
quantitation can only be achieved using this pipeline and cannot be performed using
the standard probe generation and quantitation tools.
The counts produced are by default corrected for the total number of sequences in
the dataset. Also by default they are log transformed to form an easier
distribution to work with. You can optionally correct for the length of the exons
in the transcripts to produce RPKM values. It's worth noting that although this
type of RPKM calculation is widely used in the analysis of RNA-Seq data it is not, on
its own, an ideal method of quantitation and can suffer from a number of different
biases. You should look carefully at the distributions of values you see in your data
(using for example the Cumulative Distribution Plot) to decide if further normalisation
of your data using the quantitation tools would be appropriate before filtering your
data.
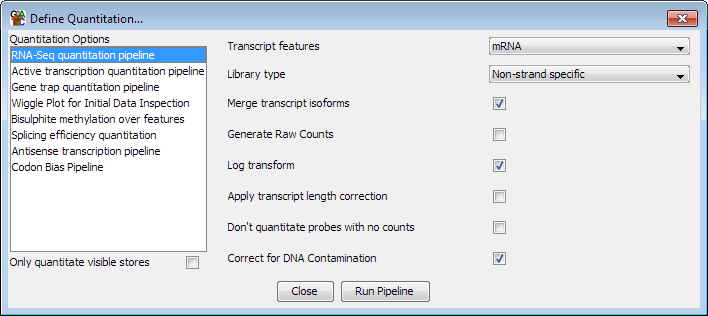
Options
The options you can set for this pipeline are:
- The feature type to use for this analysis. This will default to mRNA if an mRNA
track exists in your genome, but can be changed to whichever transcript feature track
is appropriate
- The type of library you are quantitating. Some RNA-Seq libraries are strand specific
and in these cases the pipeline can ignore reads coming from the wrong strand. You can
also choose between strand specific libraries which produce reads on the same strand as
the feature or the opposing strand.
- Whether your libraries are paired end. Since the import of RNA-Seq data is per read
then this will simply divide your read counts by 2 to produce fragment counts so you
don't over-estimate the significance of your results.
- Whether to merge the isoforms for each gene into a single measure. Using this option
relies on the transcripts using the standard Ensembl notation of gene-xxx (where xxx is
a number) to denote transcripts. The exons from all transcripts will be merged and a
single probe will be generated over the full extent of the gene.
- Whether to correct for DNA contamination - if you have DNA contamination in your
libraries it can systematically bias the counts you get, and especially affects the
quantitation of low-expressed genes. Turning this option on uses the density of reads in
intergenic regions to estimate DNA levels and then applies this as a correction to each
transcript to try to give a more accurate overall count coming specifically from
transcription.
- Whether to correct for duplication. This method is only really relevant when you are
generating raw counts, but can be useful when using count based statistics on duplicated
data. It calculates the most likely global duplication level for the data and divides the
per read counts by this value when collating the initial counts. The value is decided in
two steps. To get a value >1 the total of reads with counts of 1 must be less than the
combined counts of reads with duplication levels of 2 to 10. The actual duplication level
is then taken to be the modal duplication level (above 1) seen across all reads)
- Whether to generate raw counts. If you are using this option to generate data to put
forward for statistical analysis by a count based method (eg DESeq, EdgeR etc), then you
will need completely raw uncorrected counts. Selecting this option disables all correction
and normalisation and produces raw read counts.
- Whether the results should be log2 transformed. Data analysis and visualisation of
RNA-Seq data is often easier when performed on a log scale. If this option is selected then
empty transcripts will be given a count of 0.9 bases (or 0.9 reads if read length correction
is applied). This count is applied before read length or total read count correction is applied.
- Whether to correct for the length of each transcript. If this option is selected then
the quantitated values are expressed per kilobase of transcript. This option is only useful
if you need to compare expression levels between multiple transcripts in the same sample. If you
want to compare expression between different datasets then you should generally not select this
option since it will cause the error profile for your data, which is generally correlated with
the level of observation, to become confounded with the length of the transcript - making it
harder to accurately identify differentially expressed transcripts.

